The techniques of bone grafting are constantly evolving in dentistry with the aim of achieving bone and tissue regeneration with reduced morbidity, cost, and treatment time.
The first generation of platelet-rich aggregates included platelet-rich plasma (PRP) and platelet-rich growth factor (PRGF). On the other hand, PRF stands for platelet-rich fibrin, which undergoes natural coagulation. The use and obtaining of PRF are simpler, require less time, do not require the use of anticoagulants, as well as artificial additives that influence the coagulation cascade such as bovine thrombin and calcium chloride. The use of leukocyte-platelet-rich fibrin (L-PRF) has become an alternative treatment in cases where there is a need for regeneration, both in soft and hard tissues. This second generation of platelet concentrates, extracted from the blood and obtained after processing through centrifugation, was introduced by Choukroun and colleagues in 2001.
The protocol for obtaining L-PRF involves collecting the patient’s own blood by venipuncture, in 9-10mL tubes coated with glass/plastic that must be immediately centrifuged at high speed at 400g RCF: 2700 rpm for 12 minutes or 3000 rpm for 10 minutes. After centrifugation, the constituents of the blood separate into three layers.
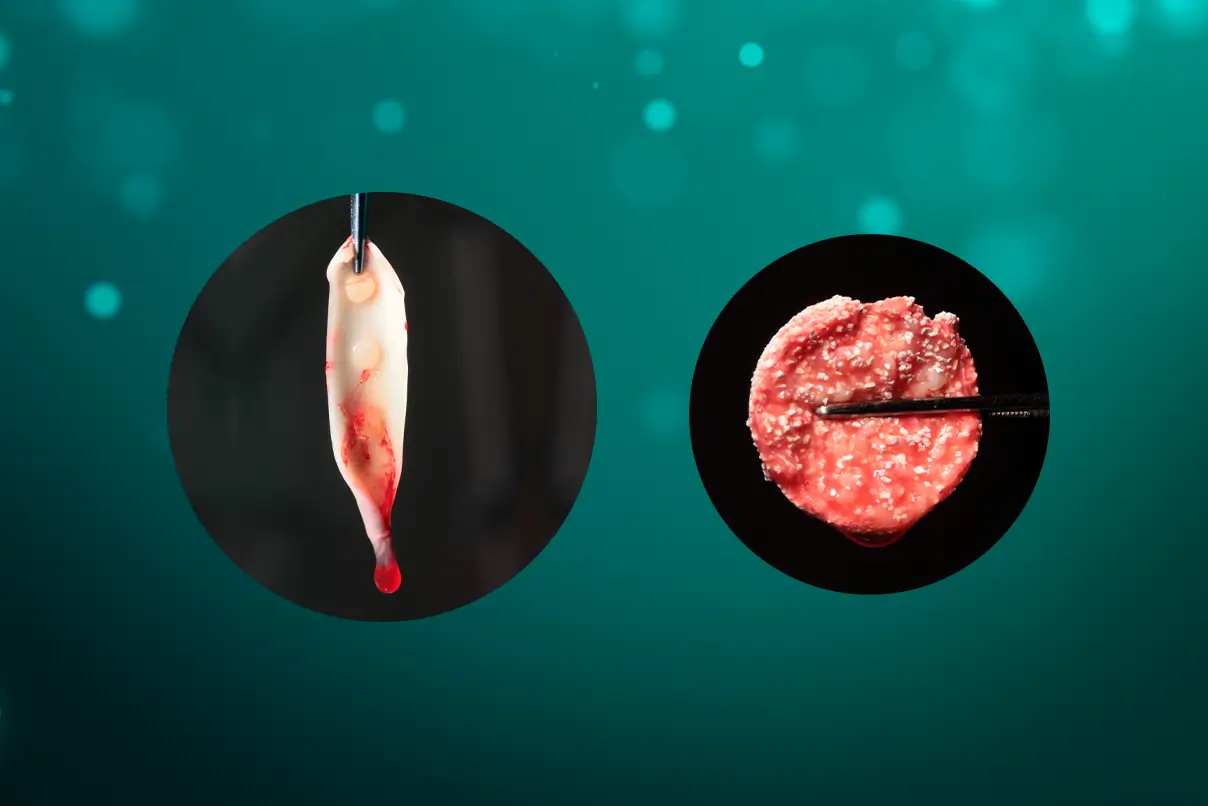
At the bottom of the tube, red blood cells accumulate, in the upper part, there is platelet-poor plasma, and the intermediate layer consists of the highest concentration of platelets and leukocytes (fibrin clot that we call L-PRF), as shown in Figure 1.
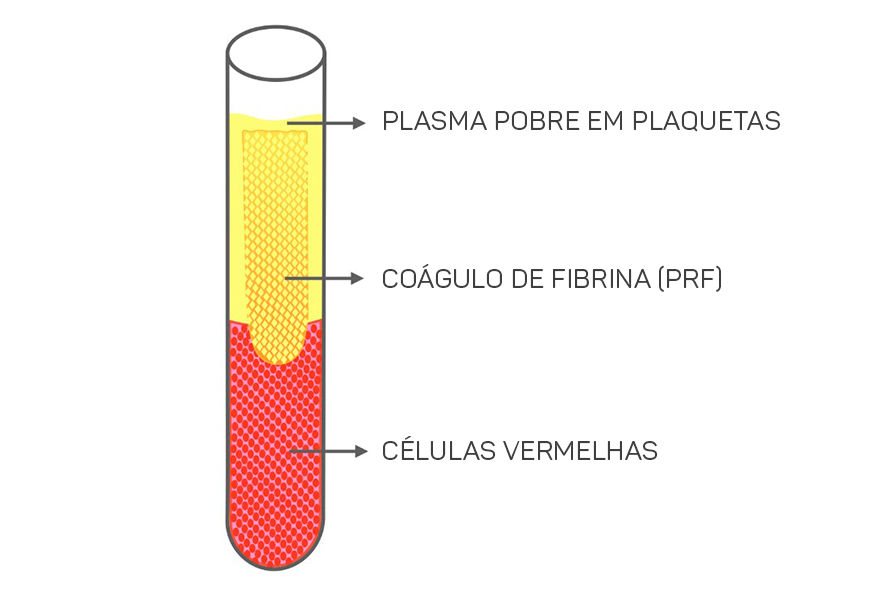
In a matter of minutes, the activation of the majority of platelets in the blood sample occurs due to contact with the glass walls of the collection tube, triggering the coagulation cascade.
The L-PRF clot is rich in fibrin, platelets, leukocytes, monocytes, and growth factors. Studies suggest a positive effect on cell proliferation, migration, adhesion, differentiation, and the inflammatory process, which justifies its widespread use in dentistry.
How to use clinically?
For the clinical use of L-PRF, the clot is removed from the tube with forceps, and the portion of red blood cells adhered to the clot is then removed using a spatula, scissors, or a scalpel blade.
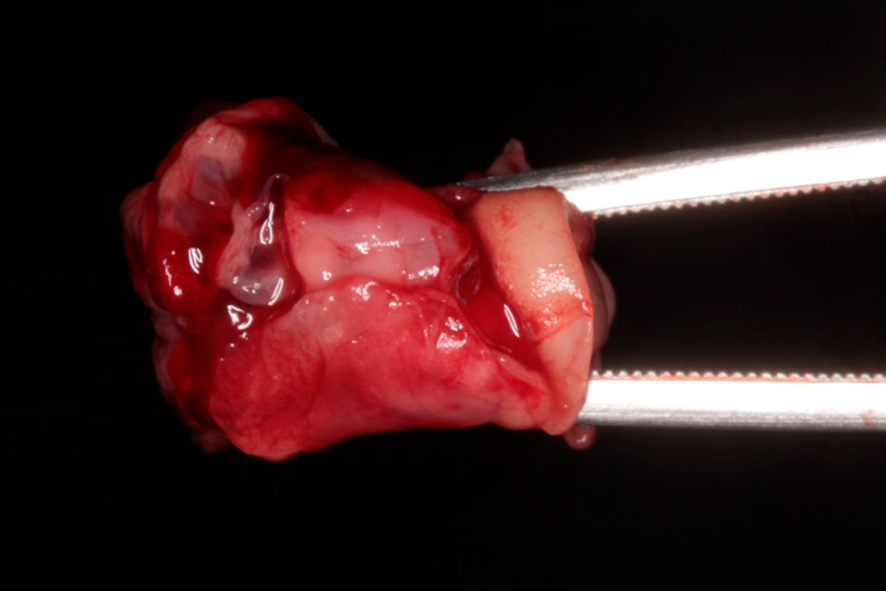
The fibrin clot can be transformed into plugs by compression, which can be used, for example, to seal extraction sockets (figure 2), or membranes with a thickness of 1mm. It can also serve as a framework for binding bone substitute particles, such as Nanosynt, for instance.
For membrane formation, the fibrin clot is placed on a perforated surface inside a metal box (PRF box) (figure 3), and it is compressed by the force of gravity with the assistance of the metal plate of the box for a period of 5 minutes.
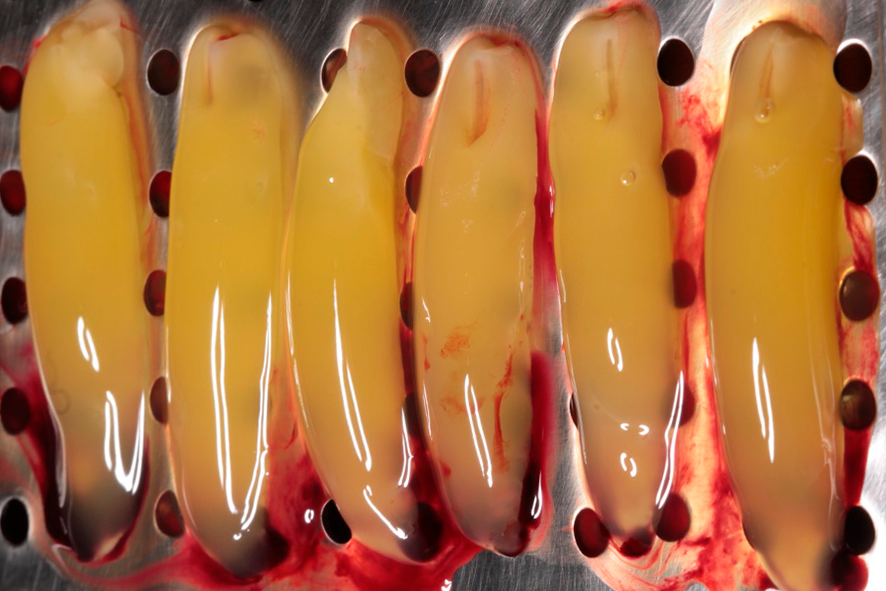
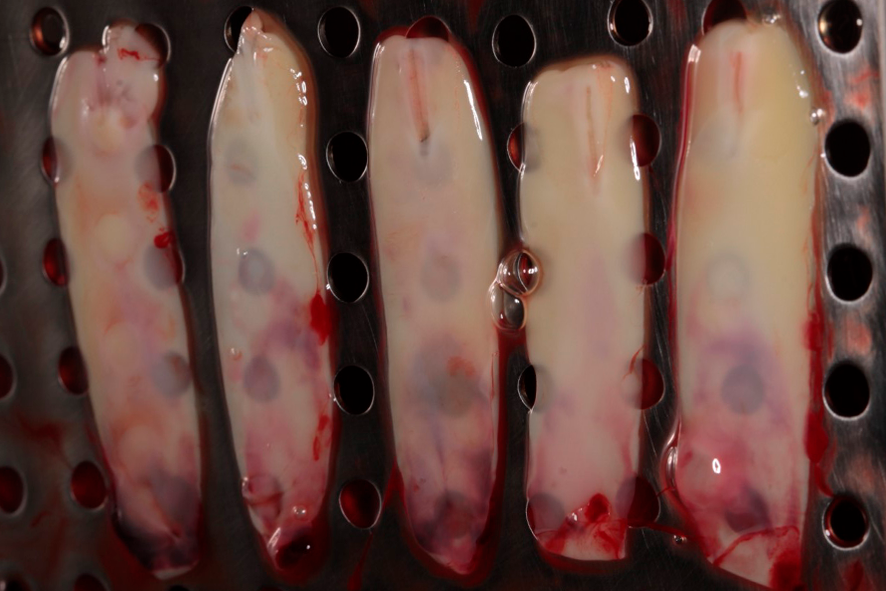
The exudate released by the clot, due to its compression, will be stored in the lower compartment of the box. This exudate is rich in proteins (fibronectin and vitronectin) and can be used to hydrate grafts and biomaterials, as well as to irrigate surgical wounds. Figures 4 and 5 show the membranes after compression, ready for use, which can be employed to cover the palate that served as the donor area for gingival graft, as well as in the recipient area to aid in the formation of keratinized tissue.
 |
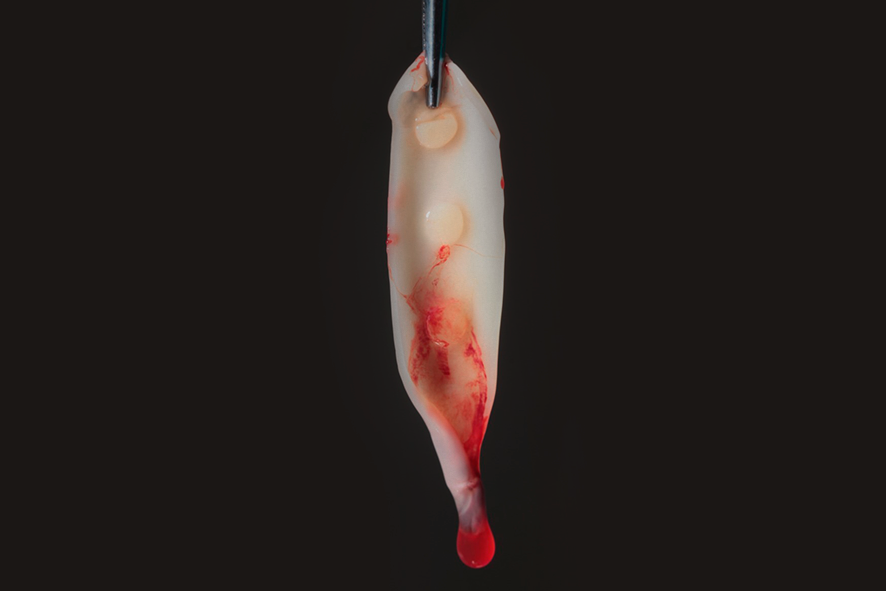 |
Figures 4 and 5 – In figures 4 and 5, we observe the membranes after compression, ready for use. At this stage, they can be used in a diced form with the graft – sticky bone, or as membranes, covering grafts, donor areas, and recipient areas.
Regarding collagen membranes in bone grafting techniques; in the maxillary sinus for sinus lifting procedures, and in conjunction with the sticky bone technique, which will be described below. As for the preparation of the plug, it involves placing the fibrin clot into the small cylinder of the PRF box and gently pressing it with a cylindrical weight. Both the membrane and the plug can be used within a period of 2.5 to 3 hours after collection, and they should be irrigated with the exudate to prevent dehydration.
L-PRF can be used in combination with biomaterials or to agglutinate bone particles, forming a block called PRF block or sticky bone (figure 6).
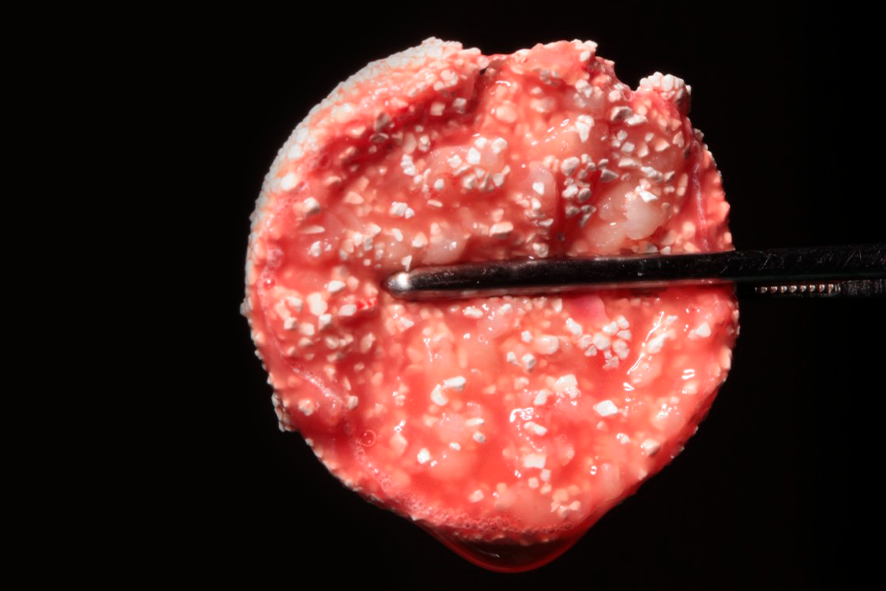
This technique increases the volume for reconstructing large defects, reducing the amount of required biomaterial and consequently the procedure’s cost. To prepare the PRF block, the patient’s blood is collected in 6 tubes coated with glass and 2 tubes coated with plastic. Plastic-coated tubes do not initiate the coagulation cascade, keeping fibrinogen in a liquid state. These tubes should be removed after 3 minutes of centrifugation, while the others should be kept until the cycle is complete. The fibrinogen (yellow liquid inside the plastic-coated tube) should be removed from the portion closest to the red blood cells, which are deposited at the bottom of the tube, using a plastic pipette, without aspirating the cells. The liquid should be kept inside the syringe. After the centrifugation of the other tubes is complete, the L-PRF clots are removed and gently compressed, as mentioned earlier, to form L-PRF membranes. In a glass or metal container, two membranes should be diced into small fragments using scissors and mixed with 0.5g of a bone substitute until a uniform mixture is obtained. 1cc of liquid fibrinogen (i-PRF) should be added, gently mixed for about 5 seconds, and the L-PRF block molded.
L-PRF has been indicated in periodontal plastic surgery, jaw osteonecrosis, buccal sinus communication, infra-bony defect regeneration, alveolar preservation, maxillary sinus lift, furcation lesions, gum recession, implant surgeries, and proves to be an excellent adjunct in the regeneration process.
REFERENCES
Choukroun, J. (2001) Une opportunit_e en paroimplantologie: le PRF. Implantodontie, 42, 55– 62. French.
Dohan, D. M., Choukroun, J., Diss, A., Dohan, S. L., Dohan, A. J., Mouhyi, J. & Gigly, B. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101: E37–44.
Pan J, Xu Q, Hou J, Wu Y, Liu Y, Li R, Pan Y, Zhang D. Effect of platelet-rich fibrin on alveolar ridge preservation: A systematic review. J Am Dent Assoc 2019; 150: 766-78. doi: 10.1016/j.adaj.2019.04.025.
Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W,
Quirynen M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol 2017; 44: 67–82. doi: 10.1111/jcpe.12643
Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W,
Quirynen M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation, and implant therapy. A systematic review. J Clin Periodontol 2017; 44: 225–234. doi: 10.1111/jcpe.12658
Temmerman A, Vandessel J, Castro A, Jacobs R, Teughels W, Pinto N, Quirynen M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol 2016; 43: 990–999. doi: 10.1111/jcpe.12612.