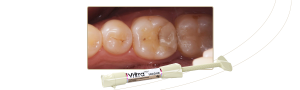
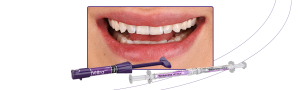
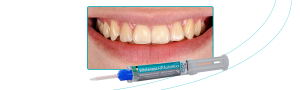
FGM Dental Group, pioneering dental industry company from Santa Catarina, Brazil, and present in more than 100 countries, celebrates an impressive achievement: obtaining the MDR certification (EU Medical Device Regulation 2017/745). This milestone represents a strategic step for the company, reinforcing its commitment to quality, safety, and innovation in its products.
The company obtained certification for many products in its line, a strict process involving more than 10 group of items, including composites, cements, adhesives, fiberglass posts, implants, abutments, drill, and others are covered by this certification.
Known as one of the most rigorous international regulations for medical devices, the MDR certification is an essential requirement for the commercialization of products in the European market and various other countries that adopt this standard.
“MDR certification is a milestone that reaffirms our commitment to excellence, safety, and innovation in our products. In addition to expanding our presence in the European market, this achievement strengthens the trust of customers and partners in FGM. We remain dedicated to offering safe and high-performance dental solutions for professionals worldwide”, emphasizes Bianca Mittelstädt, founder and CEO of FGM.
The certification process involved rigorous audits of FGM’s manufacturing facilities and technical documentation of its products, ensuring compliance with the most up-to-date international guidelines. With a constant focus on innovation and quality, the company remains at the forefront of the sector, consolidating its presence in the global market.